One of the most classic science fair projects you can do is learn how to make a lemon battery. In fact along with volcanoes this is one of the standards I see at the science fair each year and for good reason, it’s incredible to use chemistry to generate an electric current! Today I’m going to show you how to make a lemon battery and it’s cousin a “Lime Light.” I’ll also walk you through how to turn this classic project into a science fair experiment!


This post contains affiliate links.
This project is featured in my book STEAM Play & Learn! It’s a wonderful project for parents and kids to do together. To see my book and more easy STEAM project for preschool aged kids and up hop over here:

How to Make a Lemon Battery
Lemon Battery/Lime Light Materials

- (6-7) Lemons/Limes
- Galvanized nails (Find these at the hardware store)
- Copper Wire
- Wire Cutters
- 6V LED
- Electrical Tape
- Alligator Clips (optional but recommended)
- Voltmeter
Lemon Battery & Lime Light Instructions
Time needed: 20 minutes.
Learn how to make a lemon battery. Limes may be used interchangeably in this project. I like to call it a Lime Light!
- Cut your wire
Cut a 2” length of copper wire.
- Place wire in lemon/lime
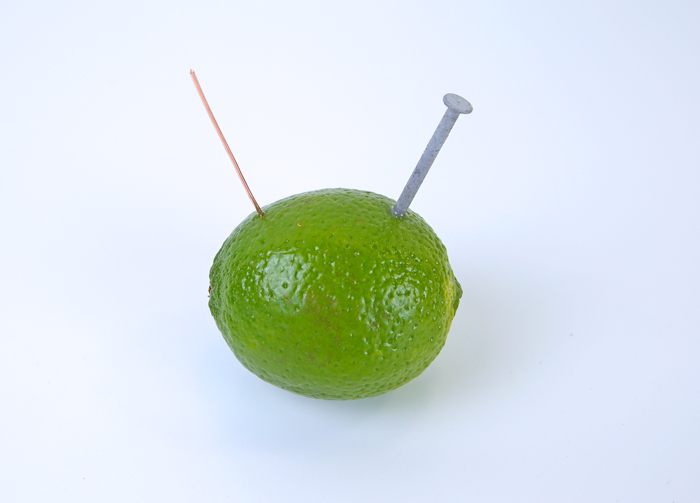
Stick the end of the wire into one side of the lemon.
- Add the nail
Place a galvanized nail in the lemon/lime approximately one away inch from the wire.

- Test it!
If you have a volt meter, connect the red test lead to the nail and the black lead to the copper wire with binder clips. You should see a reading of almost 1 volt.

- Make more “batteries” in a series
To make enough power to turn on a 6V LED you will need to make more batteries.* To make a series of (6) connected lemon batteries cut (6) roughly 3”-4” long pieces of copper wire. Wrap each piece of wire around a galvanized nail. Leave 2” of the nail unwrapped.

- Start with your first battery in the series
Place one copper wrapped galvanized nail in the first lemon/lime. Place a 2″ piece of copper wire in the other end of the lemon/lime. If you are reusing your single battery from steps 1-3, remove the galvanized nail and replace it with a copper wrapped nail. Keep the unconnected piece of copper wire on one side of the lemon/lime.
- Connect a second lemon
Place the unwrapped end of the copper wire around the nail in a second lemon/lime. Be sure to press it into the lemon flesh.

- Add a nail and repeat
Add a copper wrapped nail into the second lemon/lime and place its free copper end into a third lemon. Repeat until you have 6-7 lemons/limes in a series. Make sure they are placed in a circular shape. The seventh lemon/lime should end with an unwrapped nail in it.

- Turn it into a “Lime Light”
Take your LED and spread the leads apart. Connect one end of an alligator clip to the long (positive) leg of the LED and the other end to the copper wire in the first lemon/lime. Connect a second alligator clip to the shorter (negative) leg of the LED and the other side to the galvanized nail in the last lemon/lime. The LED should turn on! If you don’t have alligator clips use electrical tape to secure the long (positive) leg of the LED to the copper wire in the first lemon/lime and the shorter (negative) leg of the LED to the galvanized nail in the seventh lemon/lime.

Troubleshooting
- If you have a voltmeter, test the circuit to see where the power is stopping. Usually a connection is reversed or not fully inserted into the lemon.
- If the LED does not turn on reverse the LED legs. Be sure to check the connections in each lemon/ lime to make sure they are secure.
How big can you go?
At our Camp STEAM we attempted to make HUGE citrus battery by combining each of our camp team’s 6 cell battery together. We were able to get a read of 24 volts on our voltmeter with this battery! Unfortunately we also discovered it would not power a 12 V alarm and were not sure why.
One of our favorite science channels on YOuTube also tried to make GIANT lemon battery with hundreds of lemons but it also did not work as expected. Check out Mark Rober’s video here.
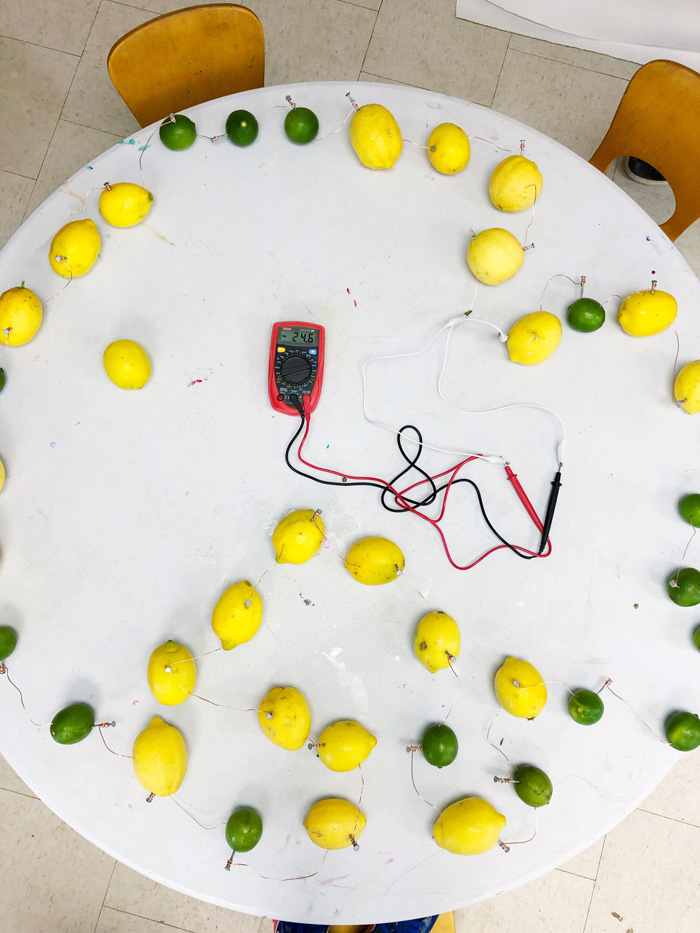
Let’s Talk STEAM
The Science: Batteries have three parts, a positive side called a cathode, a negative side called an anode, and a material in between called an electrolyte. A chemical reaction occurs in batteries causing electricity to build up in the anode until it forced to flow out of the battery in one direction.
In our lemon/lime battery, the nail acts as the cathode, the copper acts as the anode and the lime is the electrolyte. The two different types of metals, a zinc nail and a copper wire cause a chemical reaction that creates a flow of electricity.
The Technology: Our lemon/lime batteries each only make 1 volt of power but when you connect 6-7 of them together you can generate the 6 volts of electricity. This is a simple use of a chemical reaction to create enough power to turn on a light.
Turn it into a science fair project

Let’s turn this topic into an actual experiment! Here’s how you can take this to the science fair:
- Ask yourself questions How can I increase the power I get from each lemon battery? What happens if I add more nails and more wire? Can I power a motor or small alarm with a lemon battery? Do other citrus fruit work in this experiment? Do other fruits or tomatoes work? How to regular batteries work and how are they similar and different to a fruit battery?
- Research Do research online and at the library to try and predict the answer to your question. Read about how voltage is calculated. Read about the chemical reaction occurring within each lemon battery to understand the type of power it is generating.Read about the metals zinc and copper to understand why they react with citric acid. Research other fruits or vegetables that might also contain citric acid.
- Make a hypothesis A hypothesis is your prediction of the answer to your question based on your research. It may or may not be true.
- Experiment! Test your hypothesis by testing the variables and documenting them. Be sure to take notes of each experiment and what happens; this is called your data.
- Draw a conclusion Based on your experiments form a conclusion. Was your hypothesis correct?
- Share your findings Create a presentation with your findings. Include your research, hypothesis, the data you collected, and your conclusions. Be sure to include images and samples!
Experiment Examples:
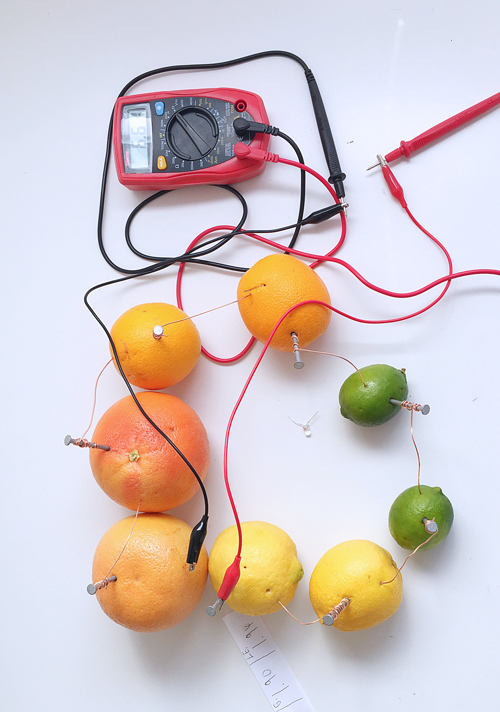
Try other fruits and vegetables Experiment with other citrus fruits to see how much power they generate. Try other acidic vegetables and fruits such as tomatoes, apples, and potatoes. Document which produce the most and least or no current. Try making a large battery from a series of different fruits and vegetables.
Try making lemon batteries with multiple nails and wires. Does putting two or more nails and copper wires in one lemon have an effect on the voltage?
What else can you power? Besides powering an LED can you make a small motor move or a small alarm go off? If not why?
More
If you enjoyed this electronics project for kids here are some more ideas:
Are you passionate about raising creative kids?
Join over 22,179 parents and educators who want connect with kids and nurture their creative process through magical, easy projects you can do TOGETHER.
Subscribe to our email list to receive project ideas as well as offers for some our creative products.
If you want to read our privacy policy before subscribing, hop over here.








Awesome post– I love this classic battery demonstration, and what a fun challenge to connect the lemon batteries together to drive up the voltage!
24 volts is impressive, but the current generated by lemon batteries is very low– that’s probably why the big lemon battery wouldn’t power the motor.